Par Mark Van Zuilen
Mark van Zuilen est géologue, actuellement à l’Institut de Physique du Globe de Paris
Introduction
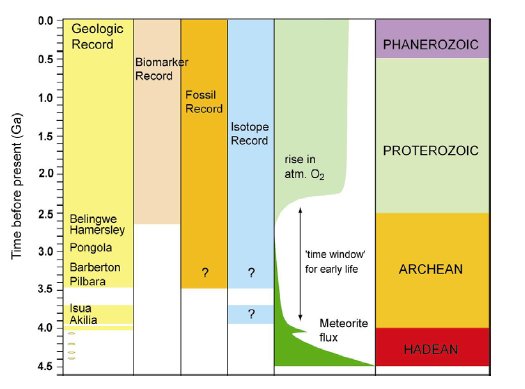
The history of life in Proterozoic and Archean times has been recorded by scarce microfossil evidence (1, 2), macrofossil evidence in the form of stromatolites (1, 3-5), molecular biomarkers (6, 7) and stable isotope ratios (8, 9) (Fig.1).
.
Figure 1. A simplified overview of the record of life on Earth over time. Episodes of high meteorite flux to the Earth during the Hadean ceased after ca. 3.9 Ga. Life did not exist or was frequently destroyed during this time (10). The rise in atmospheric oxygen at ca. 2.4 Ga (11) was caused by oxygenic photosynthesizing bacteria, which are relatively highly evolved organisms. These two events therefore define the ’time window’ for the origin and evolution of early life on Earth. Metamorphic alteration of rocks between 2.7 ga and 3.9 Ga have greatly complicated the study of the early evolution of life on Earth. Unambiguous biomarker evidence, in the form of molecular fossils, can only be traced until 2.7Ga ago (6, 7). In older rocks metamorphism has destroyed molecular biomarkers and has caused ambiguity (indicated by question marks) in the interpretation of microfossil and carbon isotopic evidence for life.
In the progressively metamorphosed rock record of the Early Archean all of these indicators have been found to be ambiguous. Possible microfossil structures have lost most of their original morphology, organic compounds including molecular biomarkers have turned into kerogen or crystalline graphite (of uncertain origin), and isotope signatures have been blurred by exchange reactions and hydrothermal processes. Furthermore, several abiologic metamorphic reactions have been identified that can produce kerogen or graphite (12, 13), and specific abiologic processes have been described that can generate complex structures that resemble microfossils (14). These problems have led to several ongoing controversies regarding life in the Early Archean. Some of these controversies are discussed below.
Carbon isotopes
Carbon isotope ratios have been used extensively to trace back life over the geological record (e.g. (9) and references therein). The carbon isotope ratio δ13C is expressed as δ13C= ([(13C/12C)sample/(13C/12C)ST]-1)*1000 in per mil (‰), relative to a standard (ST= Vienna Pee Dee Belemnite, VPDB). The two main reservoirs of carbon in sediments are carbonates with an average δ13C of 0 ‰, and the remains of biologic material with δ13C values of -25 ‰ on average. This characteristic difference in isotope ratio between the two carbon reservoirs has been observed in many organic-rich sediments of different ages, and is due to a kinetic isotope effect associated with irreversible enzyme-controlled metabolic pathways of autotrophic organisms (most of them photosynthetic). Processes that can change the original carbon isotope ratio in highly metamorphosed rocks include isotope exchange with carbonates or CO2-rich fluids (15, 16) and devolatilization reactions during metamorphism (17). These processes shift the δ13C of sedimentary biological material to higher values, making it isotopically indistinguishable from e.g. graphite that forms abiologically during metamorphic processes (13, 18).
3.8 Ga : The Isua Supracrustal Belt, West Greenland
Early work on the 3.8 Ga old Isua Supracrustal Belt (ISB) in southern West Greenland showed evidence for a marine depositional setting ; banded iron formations (BIFs), metacherts, pillow lava structures, carbonates, and felsic metasediments in which graded bedding is locally preserved (Fig.2).
.
Fig.2 Rock types in the Isua Supracrustal Belt, southern West Greenland. a) Pillow lava structures, b) Banded Iron Formation, c) Metacarbonate, d) Graded bedding (turbidite, picture from (19)).
The occurrences of siderite and dolomite, occasionally interlayered with quartzite in the ISB, appears similar to marine platform deposits that are found throughout the Precambrian and the Phanerozoic, and in early studies this field appearance led to the interpretation of a shallow marine, subtidal depositional environment (20). The ISB has a complex metamorphic history ; evidence has been reported for multiple episodes of early Archean deformation and metamorphism (21). These events were responsible for amphibolite-facies metamorphism, reaching temperatures between 500-600ºC and pressures to 5-5.5 kbar (22). Biologic remains in these sedimentary sequences would therefore have been converted to crystalline graphite. Researchers have long alleged that graphite contained in the ISB could be biogenic in origin (9, 16, 17, 23). The wide range of carbon isotope ratios (δ13C range from -25 to -6 ‰) of graphite in carbonate rich rocks has been interpreted to reflect post-depositional isotopic equilibration of graphitizing organic matter with co-existing carbonates. More recent work has shown inconsistencies in this interpretation (13, 18). Protoliths of several carbonate-rich rocks in Isua have been reinterpreted (24, 25) as secondary metasomatic and not as sedimentary in origin. This fundamental reinterpretation of the protolith would rule out a biogenic origin of graphite in metasomatic rocks. Graphite was found in large quantities in such metasomatic carbonate-rich rocks, whereas no distinguishable graphite particles were found in sedimentary BIF and metacherts (Fig.3).
.
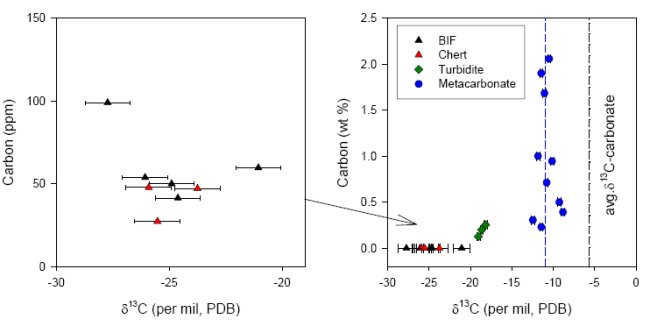
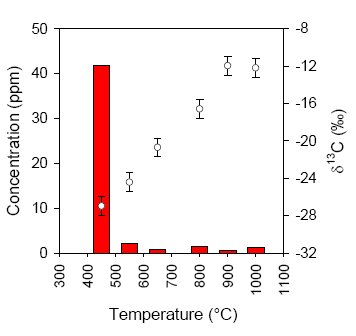
In all of the BIF and metachert samples a very low concentration of reduced carbon was measured (less than 100 ppm), that could be combusted at relatively low temperature (450°C, see Fig.4). Since graphite typically combusts around 700-800°C and all syngenetic organic material in Isua should have turned into graphite during metamorphic events, it can be concluded that the small amounts of isotopically light reduced carbon in these samples are mainly derived from postmetamorphic (and thus much younger, non-indigenous) organic material.
.
Fig.4 Example of a stepped-combustion experiment of a metachert sample (13). The concentration of reduced carbon (left axis) is determined by measuring the amount of CO2 produced during combustion. At each temperature step this CO2 is subsequently analyzed for carbon isotope ratio (right axis).
With the exception of some rare turbidite deposits (19, 27), the petrographic evaluation casts doubt on the biological origin of the graphite occurrences in the ISB. The association of graphite with MgMn-siderite and magnetite was observed in all graphitic carbonate veins within mafic country rocks (Fig.5), suggesting that graphite and magnetite in these rocks are the products of partial thermal disproportionation of the carbonate. The siderite (FeCO3) disproportionation reaction, yielding graphite and magnetite (6 FeCO3 à 2Fe3O4 + 5CO2 + C) has been studied in detail at metamorphic P,T,fO2-conditions (28), and has been suggested earlier as a possible mechanism for graphite formation in the amphibolite facies (T ca. 550ºC ; P ca. 5 kBar) ISB (29).
.
Fig.5. a) Outcrop of a metacarbonate vein within mafic country rock, eastern part of the ISB. The hammer indicates the point where this vein pinches out. b) SEM-BSE image of a metacarbonate thinsection. Mineral phases Sid= MgMn-siderite, Apa= apatite, Mag= magnetite, Gr= graphite (13).
.
Micrometer-size graphite inclusions with a pronounced light δ13C value (weighted mean -30±3 ‰ ; ion microprobe data) were reported to occur in apatite crystals from the ISB (30). The graphite was thought to have escaped isotope exchange with the associated carbonates due to armoring by the host apatite. This claim was based on a rock sample that at the time was believed to represent a sedimentary BIF. More recent petrographic analysis has revealed that it contains MgMn-siderite-magnetite-graphite associations and is compositionally akin to Isua metacarbonates (13, 31). Furthermore, the REE pattern of these graphite-bearing apatites is distinctly different from apatites occurring in sedimentary rocks (31). As is shown in Fig.5b graphite is not restricted to apatite, but occurs as inclusions in most other phases too. The petrographic and geochemical evidence strongly suggests that this graphite is produced epigenetically through thermal disproportionation of ferrous carbonate. This type of graphite consequently appears to be without biological significance and formed in one or several thermal events later than 3.8 Ga. The isotopic systematics of the process responsible for formation of isotopically light graphite (weighted mean -30±3 ‰) enclosed in apatite crystals remains to be studied, but petrographic evidence clearly excludes a primary biogenic origin.
3.8 Ga : Akilia island, West Greenland
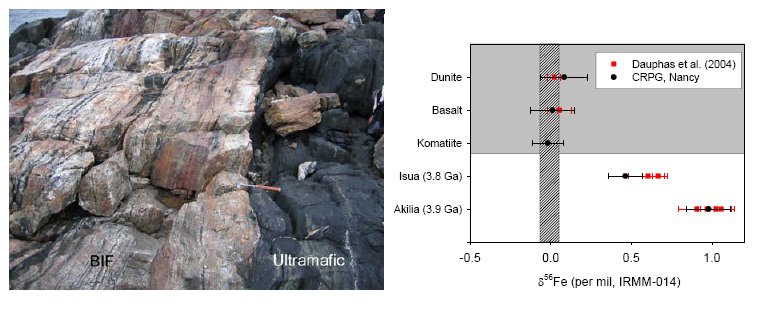
A highly metamorphosed quartz-pyroxene rock on the southwestern tip of Akilia Island has for long been the center of attention regarding the oldest traces of life on Earth. This five meter wide outcrop (Fig.6a) was interpreted as a BIF and was found to contain graphite inclusions within apatite crystals (30). The low δ13C of these graphite inclusions suggested a biologic source material that had retained it’s original carbon isotope signature. This claim has since been the center of controversy, as it was argued that the protolith of this rock was not a BIF, but instead a highly metasomatized ultramafic rock which does not represent a marine depositional setting and would not be able to harbor traces of ancient life (32). Since then, geochemical data has been presented to either argue for or against a sedimentary origin (33-35). Recently, iron isotope systematics and trace element ratios have been used to establish more firmly a sedimentary origin (36). The iron isotope ratio δ56Fe is expressed as δ56Fe=([(56Fe/54Fe)sample/(56Fe/54Fe)ST]-1)*1000 in per mil (‰) relative to a standard (IRMM-014 reference material). Igneous rocks worldwide have a near-constant δ56Fe near zero ‰, while BIFs display a wide range of δ56Fe (37). A shift towards positive δ56Fe is typically observed in BIFs that are dominated by oxidized Fe-mineral phases such as magnetite and hematite. This shift towards positive δ56Fe was indeed observed in a BIF from Isua, and subsequently in the quartz-pyroxene rock on Akilia island ((36), Fig.6b). It is of crucial importance to establish that such a positive δ56Fe is not the result of metasomatic alteration of an original igneous protolith. For instance altered mid-ocean ridge basalts (MORB) have positive δ56Fe values (38). However, such positive δ56Fe correlate with a depletion in Fe concentration. Loss of Fe would be evident in the comparison of the ratios of Fe to an immobile trace element (e.g. Ti, Nb, Hf). If a similar loss of Fe occurred as a result of metasomatic alteration of the quartz-pyroxene rock on Akilia Island, it would be evident in the comparison of the Fe/Ti ratio between this rock and the surrounding igneous rocks. A high Fe/Ti ratio in the quartz-pyroxene rock indicates that Fe was not preferentially lost. On the contrary, the high Fe/Ti ratio resembles those of BIFs found in Isua (39).
.
Fig.6 a) Overview of Akilia outcrop, b) Iron isotope ratio of Akilia outcrop, compared with Isua BIF and ultramafic rocks (data from (36) ; van Zuilen, unpublished data).
Although several lines of evidence now suggest that this rock indeed represents a sediment (36, 40), the age is still debated (41, 42), and the claim for the oldest trace of life on Earth is still strongly contested. Graphite inclusions in apatite are extremely rare (43) and it has been suggested that the apatite crystals themselves are much younger than the surrounding rock matrix (44).
3.5 Ga : Pilbara, Western Australia
Carbonaceous microstructures resembling fossilized bacteria were reported from the 3.5 Ga old Apex chert in Pilbara, Western Australia (45, 46). At the time this chert was thought to represent a shallow marine depositional setting in which photosynthetic bacteria could thrive. However, it was shown by (12) that the samples studied by (45) actually represent a hydrothermal feeder dike in an ocean floor basaltic surrounding (Fig.7). It is therefore highly unlikely that photosynthetic bacteria occurred in such an environment. Brasier et al. (12) argued for the abiologic origin of the observed carbonaceous particles by a Fischer-Tropsch type reaction (FTT) that occurred during hydrothermal serpentinization of surrounding basalts (Fig.8). It has since been suggested, however, that the structures in the Apex chert represent the remains of chemoautotrophic bacteria that lived in such a hyrothermal environment, directly metabolizing gases emanating from seafloor volcanism (47). Alternatively, it is suggested here that FTT-reactions themselves could have produced methane and other low molecular hydrocarbons on which these organisms feeded. The processes of stable isotope fractionation associated with Fischer-Tropsch type reactions are still largely unknown. Carbon ratios may be affected in a way that is distinguishable from biologic processes. Furthermore, it is unknown to what extent heteroatoms like nitrogen or sulfur are incorporated in organic products during these abiologic reactions.
.
Fig.7 Outcrop of the Apex Chert near Chinaman Creek, Pilbara in Western Australia. Microfossil structures described by (45, 46) occur in a hydrothermal feeder dike, not in the actual sedimentary portion of the Apex Chert.
Fig.8 Schematic of hydrothermal feeder dike (background drawing from (48)). Hot fluids circulate the basaltic ocean floor and cause serpentinization, and precipitate silica when cooled. Fischer-Tropsch-type reactions associated with serpentinization produce methane and low molecular weight organics that could have been a source of energy for lithoautotrophic bacteria. Alternatively, high-molecular weight organics and kerogen could have been produced directly by Fischer-Tropsch type reactions, providing an entirely abiologic explanation of carbonaceous structures within hydrothermal feeder dikes.
.
3.4-3.2 Ga : Barberton, South Africa
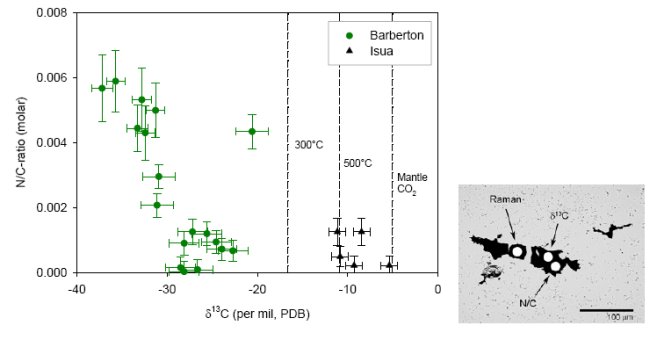
The problems interpreting δ13C in highly metamorphosed terrains have prompted the search for additional chemical and isotopic biomarkers. Metamorphosed biological material (e.g. kerogen, coal, solid bitumen, shungite) still contains small amounts of heteroatoms like H, O, N and S. During high-grade metamorphism such materials would progressively loose these heteroatoms, obtain a higher degree of structural order and would ultimately transform into crystalline graphite. Current research is focused on combining δ13C analysis with the analysis of heteroatom concentration. Carbonaceous structures occur in cherts within the 3.4-3.2 Ga Barberton Greenstone Belt, South Africa, that have been interpreted as microfossils (49, 50). These cherts have experienced regional greenschist facies metamorphism, and local hydrothermal alteration, which would have altered sedimentary biologic material to kerogen and would have caused loss of heteroatoms and shifts in δ13C. Using an ion microprobe, in-situ analysis of δ13C and N/C-ratio was carried out on individual carbonaceous microstructures (Fig.9). In addition, individual structures were analyzed by Laser-Raman spectroscopy. For comparison the N/C-ratio and δ13C value of Isua graphite are shown as well. The graphite sample from Isua has virtually no nitrogen. The average δ13C value reflects the expected equilibrium isotope fractionation delta13Cgr-CO2= -6.4 ‰ at 550°C (26) between graphite and mantle type CO2 (δ13C ca. -5 ‰) which likely represents the ultimate carbon source. The chert samples from Barberton have experienced greenschist facies metamorphism. If the carbonaceous matter in these cherts formed abiologically during metmorphism and in isotopic equilibrium with mantle type CO2, then the δ13C of this material could have obtained a δ13C as low as -17 ‰ (assuming delta13Cgr-CO2= -12 ‰ at 300°C, and mantle CO2= -5 ‰). The observed δ13C is much lower than that, and an inverse correlation between N/C and δ13C is observed. The lowest δ13C values are associated with the highest N/C ratios, and likely represent the least altered remnants of a biologic source.
.
Figure 9. a) N/C-ratio and δ13C by SIMS analysis of individual microstructures in cherts from Barberton and a talc-schist from Isua. The lines indicate the isotope ratio of graphite in equilibrium with mantle-type CO2 (-5 ‰) for different metamorphic temperatures (van Zuilen et al., submitted). b) Individual carbonaceous microstructure from a chert in the Hooggenoeg Formation, Barberton Greenstone Belt. In-situ analysis is achieved by Laser-Raman spectroscopy and Secondary Ion Mass Spectrometry (SIMS).
.
Summary
From the controversies discussed above, it can be concluded that studies of early life in the incomplete and strongly metamorphosed Archean rock record face specific challenges. There is a strong need for careful description of geological context, identification of secondary metamorphic processes, and detailed structural, isotopic and chemical description of microstructures that are indigenous to and syngenetic with the rock formations. Currently it is difficult to declare with certainty what the oldest trace of life is, and importantly what it’s nature and habitat were. Several macroscopic stromatolite structures have been reported from Pilbara, West Australia and from Barberton, South Africa (1, 5). However, alternative abiologic mechanisms of formation have been suggested (51, 52). The most intriguing claims for early life now rest on graphite inclusions from a 3.8 Ga old turbidite deposit in Isua (19), kerogenous microstructures in 3.5 Ga old cherts from Pilbara (47), kerogenous microstructures in 3.4-3.2 Ga cherts from Barberton (49, 50), and several stromatolite outcrops in Pilbara (53). Unambiguous traces of life, in the form of molecular biomarkers, are found in the 2.7 Ga Hamersley Range, West Australia (6, 7), and suggest the presence of highly evolved oxygenic photosynthesizing bacteria.
Acknowledgements
Aivo Lepland (Norwegian Geological Survey, Norway), Nicolas Dauphas (University of Chicago, USA), Marc Chaussidon (CRPG-CNRS, France), Bernard Marty (CRPG-CNRS, France), Béatrice Luais (CRPG-CNRS, France), Claire Rollion-Bard (CRPG-CNRS, France), Laurent Richard (G2R-UHP, France), Gustaf Arrhenius (Scripps Institution of Oceanography, USA), Minik Rosing (Geologic Museum Copenhagen, Denmark), Peter Appel (Geological Survey of Denmark and Greenland, Denmark). Some parts of this work were funded by NASA Exobiology grants NAGW-1035, NAG5-4563, NAG5-12983, and some parts were funded by a Marie Curie Individual Fellowship from the European Union.
References
1. J. W. Schopf, Earth’s earliest biosphere, its origin and evolution (Princeton University Press, Princeton, New Jersey, 1983).
- 2. J. W. Schopf, C. Klein, The Proterozoic biosphere : a multidisciplinary study (Cambridge University Press, Cambridge, 1992).
- 3. R. Buick, J. S. R. Dunlop, D. I. Groves, Alcheringa 5, 161-181 (1981).
- 4. R. Buick, Science 255, 74-77 (1992).
- 5. G. R. Byerly, D. R. Lowe, M. Walsh, Nature 319, 489-491 (1986).
- 6. J. J. Brocks, G. A. Logan, R. Buick, R. E. Summons, Science 285, 1033-1036 (1999).
- 7. R. E. Summons, L. L. Jahnke, J. M. Hope, G. A. Logan, Nature 400, 554-557 (1999).
- 8. Y. Shen, R. Buick, D. E. Canfield, Nature 410, 77-81 (2001).
- 9. M. Schidlowski, Precambrian Research 106, 117-134 (2001).
- 10. C. F. Chyba, Geochimica et Cosmochimica Acta 57, 3351-3358 (1993).
- 11. A. Bekker et al., Nature, 117-120 (2004).
- 12. M. Brasier et al., Nature 416, 76-81 (2002).
- 13. M. A. van Zuilen, A. Lepland, G. Arrhenius, Nature 418, 627-630 (2002).
- 14. J. M. Garcia-Ruiz, S. T. Hyde, A. M. Carnerup, M. J. Van Kranendonk, N. J. Welham, Science 302, 1194-1197 (2003).
- 15. N. E. Kitchen, J. W. Valley, Journal of Metamorphic Geology 13, 577-594 (1995).
- 16. M. Schidlowski, P. W. U. Appel, R. Eichmann, C. E. Junge, Geochimica et Cosmochimica Acta 43, 189-199 (1979).
- 17. J. M. Hayes, I. R. Kaplan, W. Wedeking, in Earth’s earliest biosphere, its origin and evolution J. W. Schopf, Ed. (Princeton University Press, Princeton, NJ, 1983) pp. 93-134.
- 18. M. A. van Zuilen et al., Precambrian Research 126, 331-348 (2003).
- 19. M. T. Rosing, Science 283, 674-676 (1999).
- 20. E. Dimroth, in Sedimentary Geology of the Highly Metamorphosed Precambrian Complexes. A. V. Sidorenko, Ed. (Nauka, Moscow, 1982) pp. 16-27.
- 21. A. P. Nutman, M. V. R., C. R. L. Friend, V. C. Bennett, P. D. Kinny, Precambrian Research 78, 1-39 (1996).
- 22. J. L. Boak, R. F. Dymek, Earth and Planetary Science Letters 59, 155-176 (1982).
- 23. M. Schidlowski, Nature 333, 1988 (1988).
- 24. N. M. Rose, M. T. Rosing, D. Bridgwater, American Journal of Science 296, 1004-1044 (1996).
- 25. M. T. Rosing, N. M. Rose, D. Bridgwater, H. S. Thomsen, Geology 24, 43-46 (1996).
- 26. T. Chacko, D. R. Cole, J. Horita, in Stable isotope geochemistry J. W. Valley, D. R. Cole, Eds. (Mineralogical Society of America, Washinton, 2001), vol. 43, pp. 1-81.
- 27. M. Rosing, R. Frei, Earth and Planetary Science Letters 217, 237-244 (2003).
- 28. B. M. French, American Journal of Science 271, 37-78 (1971).
- 29. E. C. Perry, Jr., S. N. Ahmad, Earth and Planetary Science Letters 36, 280-284 (1977).
- 30. S. J. Mojzsis et al., Nature 384, 55-59 (1996).
- 31. A. Lepland, G. Arrhenius, D. Cornell, Precambrian Research 118, 221-241 (2002).
- 32. C. M. Fedo, M. Whitehouse, Science 296, 1448-1452 (2002).
- 33. C. R. L. Friend, A. P. Nutman, V. C. Bennett, Science 298, 917 (2002).
- 34. S. J. Mojzsis, T. M. Harrison, Science 298, 917 (2002).
- 35. C. M. Fedo, M. J. Whitehouse, Science 298, 917 (2002).
- 36. N. Dauphas et al., Science 306, 2077-2080 (2004).
- 37. C. M. Johnson, B. L. Beard, N. J. Beukes, C. Klein, J. O’Leary, Contr. Mineral. and Petrol. 144, 523-547 (2003).
- 38. O. Rouxel, N. Dobbek, J. Ludden, Y. Fouquet, Chemical Geology 202, 155-182 (2003).
- 39. R. F. Dymek, C. Klein, Precambrian Research 39, 247-302 (1988).
- 40. S. J. Mojzsis, C. D. Coath, J. P. Greenwood, K. D. McKeegan, T. M. Harrison, Geochimica et Cosmochimica Acta 67, 1635-1658 (2003).
- 41. M. Whitehouse, B. S. Kamber, S. Moorbath, Chemical Geology 160, 204-221 (1999).
- 42. S. J. Mojzsis, T. M. Harrison, Earth and Planetary Science Letters 202, 563-576 (2002).
- 43. A. Lepland, M. A. van Zuilen, A. Arrhenius, M. Whitehouse, C. M. Fedo, Geology 33, 77-79 (2005).
- 44. Y. Sano, K. Terada, Y. Takahashi, A. P. Nutman, Nature 400, 127-127 (1999).
- 45. J. W. Schopf, Science 260, 640-646 (1993).
- 46. J. W. Schopf, A. Kudryavtsev, D. G. Agresti, T. J. Wdowiak, A. D. Czaja, Nature 416, 73-76 (2002).
- 47. Y. Ueno, H. Yoshioka, S. Maruyama, Y. Isozaki, Geochimica et Cosmochimica Acta 68, 573-589 (2004).
- 48. I. Paris, I. G. Stanistreet, M. J. Hughes, Journal of Geology 93, 111-129 (1985).
- 49. F. Westall et al., Precambrian Research 106, 93-116 (2001).
- 50. M. Walsh, Precambrian Research 54, 271-293 (1992).
- 51. J. P. Grotzinger, D. H. Rothman, Nature 383, 423-425 (1996).
- 52. D. R. Lowe, Geology 22, 387-390 (1994).
- 53. M. J. Van Kranendonk, G. E. Webb, B. S. Kamber, Geobiology 1, 91-108 (2003).









merci, great article!